bazzi lab
Cell & Developmental Biology, University of Michigan Medical School
CECAD Cluster of Excellence, University of Cologne, Medical Faculty
Our research is centered on the integration of signaling pathways, cell cycle and cell fate during two patterning transitions in (I) early embryonic development & (II) skin development in the mouse, as a mammalian genetic model. To address our questions, we use state-of-the-art genetic, biochemical, time-lapse imaging and omics approaches in vivo, ex vivo or in vitro in mouse embryonic stem cells (mESCs) and primary cell culture. To mechanistically and functionally assess the candidates, we use CRISPR/Cas9 genome-editing in vivo and in vitro to engineer knockout and knockin alleles, including fluorescent reporters. Our goal is to dissect the mechanistic links between signaling pathways and cell fate in the developing mouse, which are usually coopted or disrupted in human diseases.
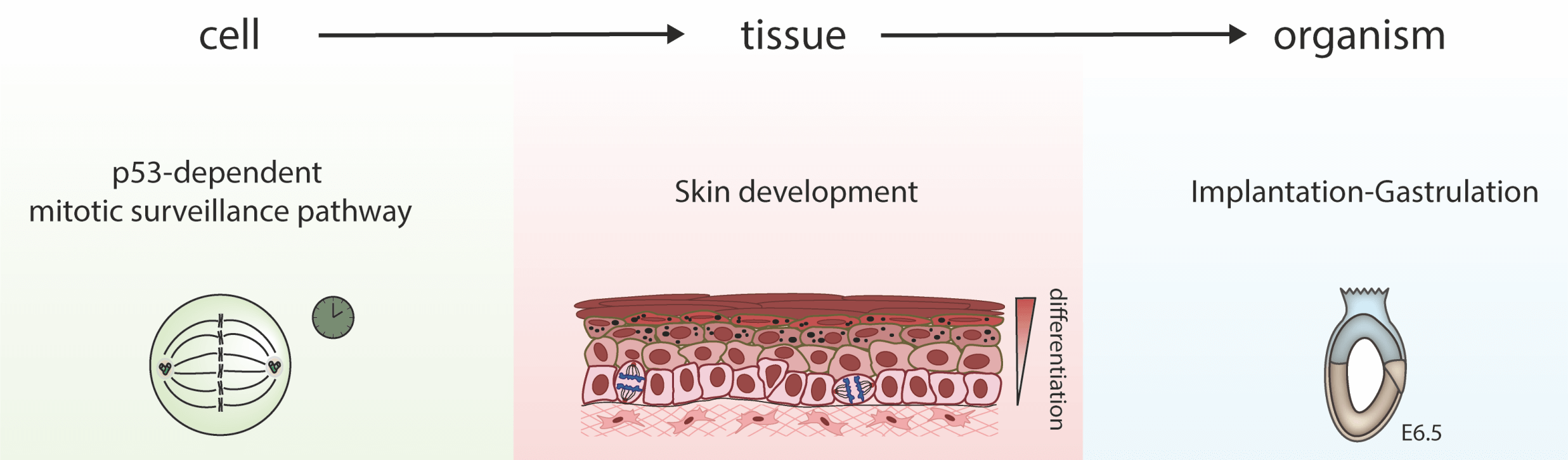
I. How do cell division and centrosomes regulate cell fate in mouse development?
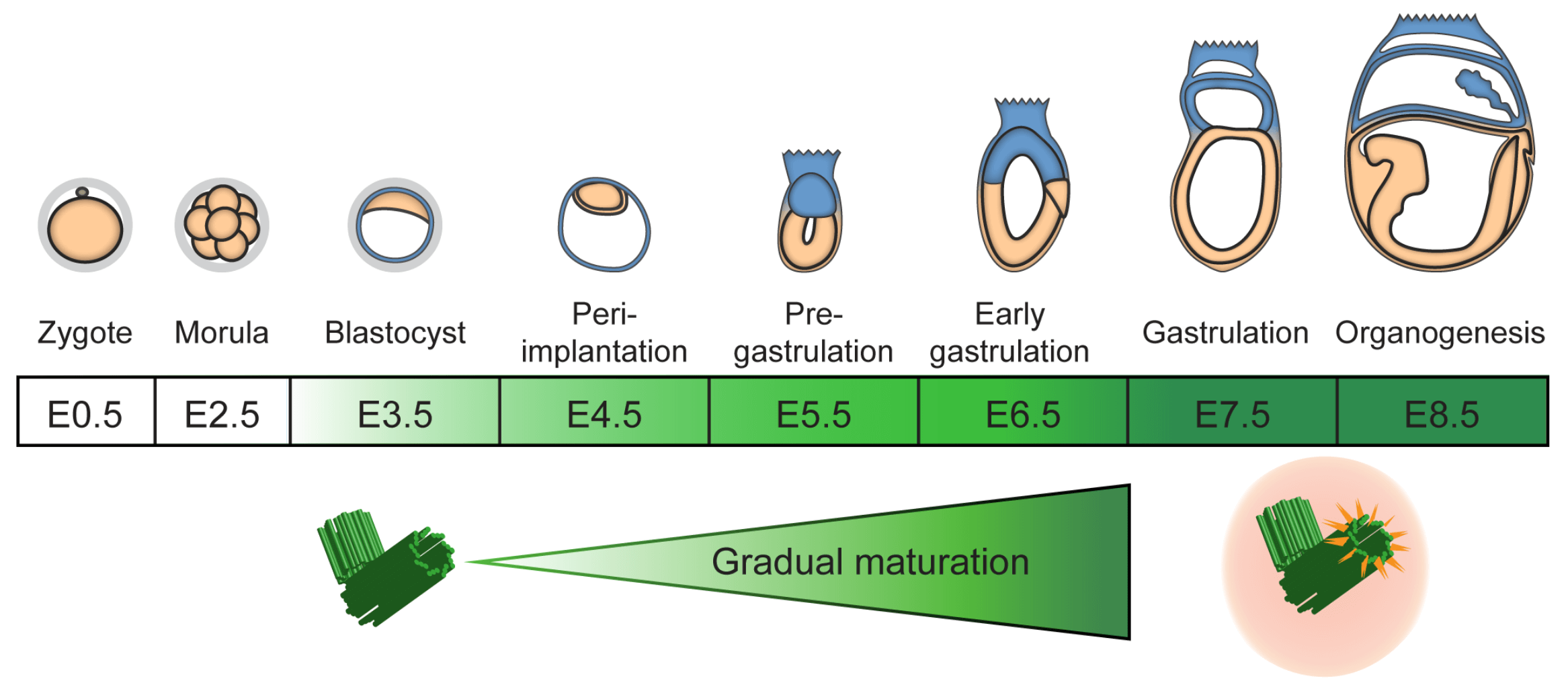
The regulation of cell division is crucial for the propagation of multicellular organisms and aberrations in proliferation are at the origin of cancer. Centrosomes, formed of a core of two centrioles and a surrounding pericentriolar matrix, are important for spindle assembly during mitosis, but also essential to provide the basal body template for cilia during interphase or in differentiated cells. Moreover, mutations in human genes encoding centrosomal proteins cause primordial dwarfism and microcephaly. Using genetic mutations in the mouse, we have previously removed centrosome function in the developing mouse embryo and brain. The main result was the activation of a p53-dependent apoptosis pathway that was not due to the secondary loss of cilia and correlated with prolonged mitosis. We and others have shown that this novel pathway is also largely independent of DNA damage or abnormalities in chromosome segregation. Our group is using genetic and biochemical approaches to unravel the mechanism of this “mitotic timer” in mouse embryos and mESCs.
Figure adapted from Xiao C. et al. (2021). Gradual centriole maturation associates with the mitotic surveillance pathway in mouse development.
II. How do signaling pathways regulate the cell cycle and fate transitions in the skin?
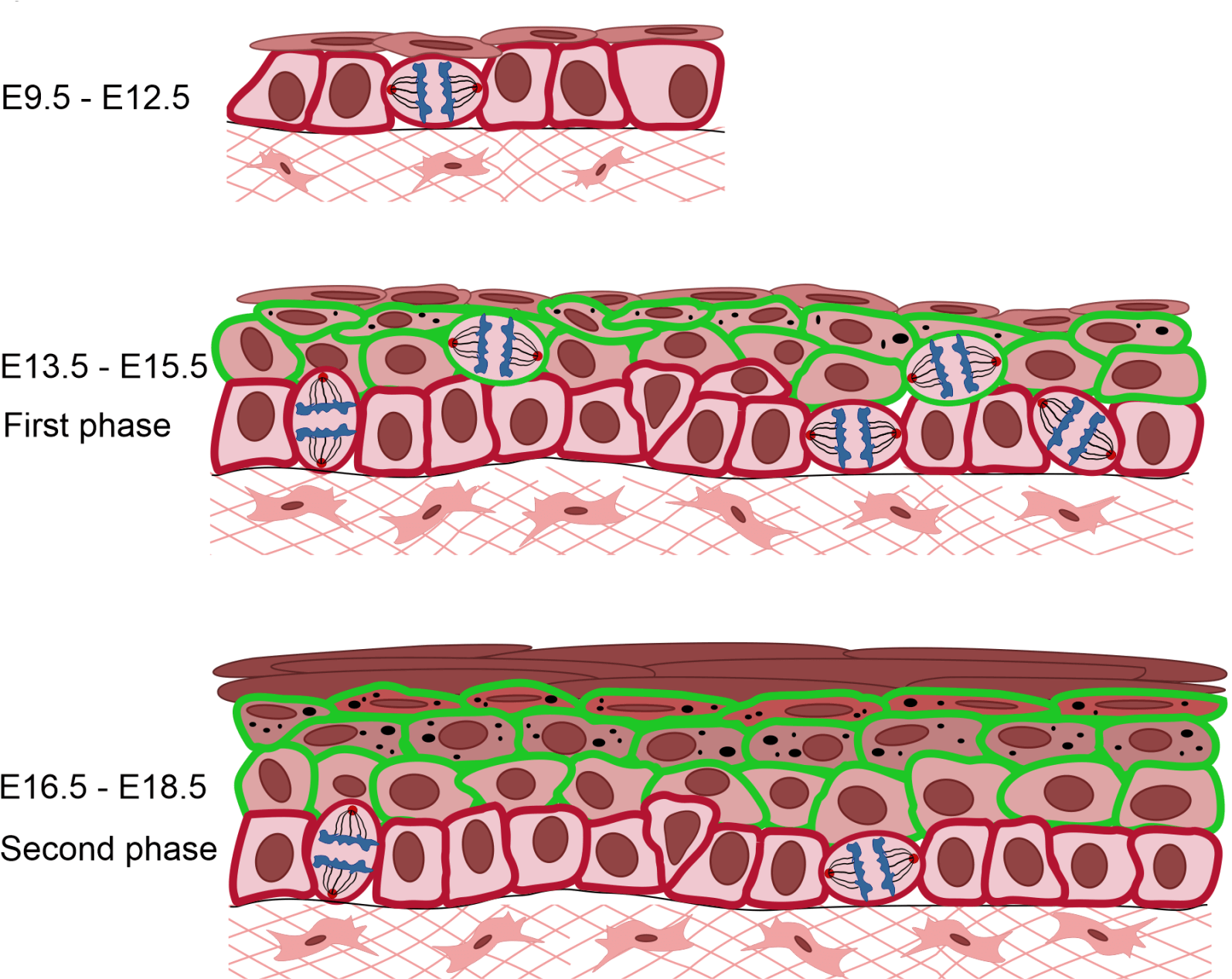
How complex stratified epithelial barriers that are essential for animal survival, such as the skin epidermis, are generated during mammalian development is still an open question. Using time-lapse imaging and mathematical modeling of the growth kinetics of the tissue, our data support a two-phase model of epidermal stratification. In the first phase, a burst of proliferation fuels the increase in cell number to promote stratification, and in the second phase, cellular delamination from the basal layer maintains the regenerative capacity of the epidermis. We are mechanistically defining the roles of signaling pathways and cell cycle regulation in these cell fate switches.
Figure adapted from Damen, M. et al., (2021). High proliferation and delamination during skin epidermal stratification.
Concurrent with epidermal stratification, the hair follciles form as well-patterned islands within the skin. We are addressing how the uniform field of progenitor cells in the skin epithelium transforms into stem cells, differentiated progeny and hair follicle-producing cells. Our working model is that the cell fates in the epidermis and hair follicle are flexible and positionally refined over time. To achieve this, we are using scRNA-Seq and CRISPR/Cas9-based genetic approaches in the mouse.

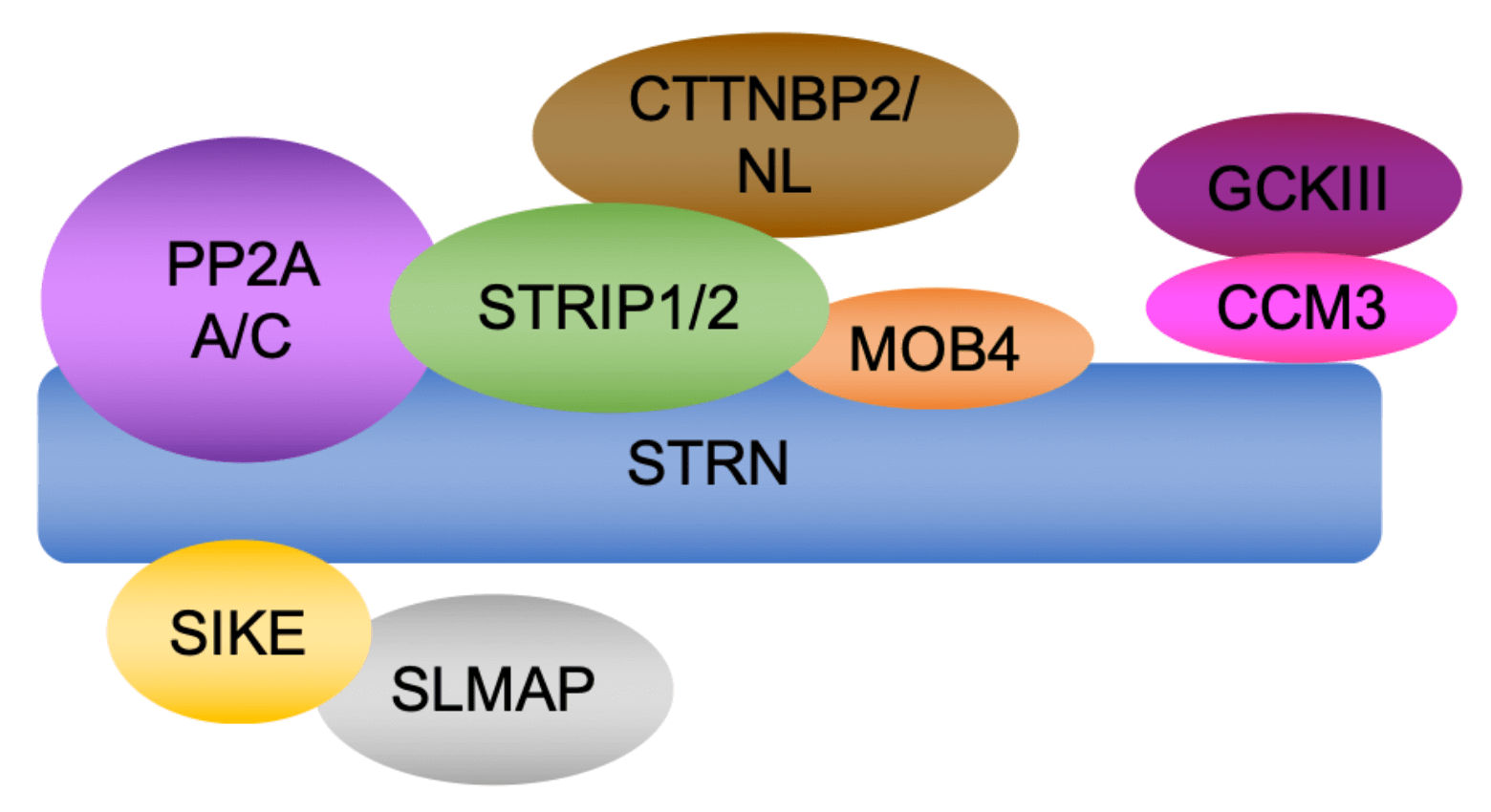
We are also interested in how the organization of other cytoskeletal elements, such as the actin cytoskeleton, impact on tissue morphogenesis and remodeling. For this, we are using mutations in the striatin-interacting phosphatases and kinases complex (STRIPAK) that regulates the activity of the phosphatase PP2A. We have shown that the STRIPAK is essential for normal mesoderm formation and migration in the mouse embryo. We are currently using the STRIPAK mutations in the mouse to define how the organization of actin governs keratinocyte and hair follicle differentiation and skin barrier formation. Our findings will have profound implications for human diseases that are associated with a defective skin barrier.
Collectively, our overarching goal is to understand how signaling pathways, cytoskeletal organization and the cell cycle are integrated with cell fate during development. Our focus on combining the biological processes from the biochemical to cell, tissue and organismal levels will help provide mechanistic target discovery in molecular biomedicine.